Heat of Fusion
The energy required to change a unit mass of a solid into the liquid state without a change in temperature is called it's heat of fusion or enthalpy of fusion.
Heat of fusion can be expressed as either calories per gram or calories per mole. The heat of fusion of water is 80 calories per gram. The energy required to melt 1 gram of ice cube at 00C to water at 00C is 80 calories or 334 Joules.
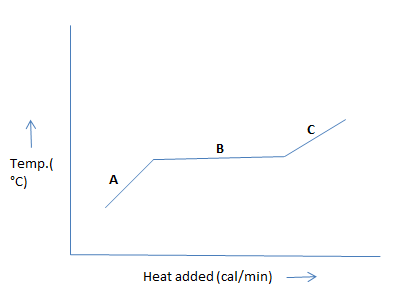
The graph below shows heat of fusion by plotting temperature against the energy requirement.
Heat of Fusion Formula can be expressed as where: q is heat energy, m is mass, ΔHf is heat of fusion.
Example:How much energy is required to melt 100 grams of ice at its melting point?