Order of filling electrons in the orbitals and Shells
When an atom or ion receives electrons into its orbitals, the orbitals fill up in thefollowing order:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s
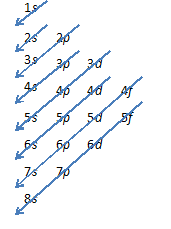

When an atom or ion receives electrons into its orbitals, the orbitals fill up in thefollowing order:
